|
The copepods are the largest and most diversified group of crustaceans. At present they include over 14.000 species, 2.280 genera and 210 families, a surely underestimated number, inhabiting sea and continental waters, semiterrestrial habitats, or living in symbiotic relationships with other organisms. They are considered the most plentful multicellular group on the earth, outnumbering even the insects, which include more species, but fewer individuals! Particularly, the copepods are the dominant forms of the marine plankton and constitute the secondary producers in the marine environments and a fundamental step in the trophodinamics of the oceans.
During their long evolutionary history, starting in the Lower Cretaceous, copepods spread over all the continents, as well as they successfully colonized about all the available water habitats of the Planet, becoming well adapted or specialized to very different salinity regimes, from marine and hypersaline waters to continental freshwater bodies, and to a wide range of temperature from the polar to the hot springs waters.
Copepods regularly live also in marine and freshwater sediments, in different groundwater substrates, in continental and anchialine caves (limestone caves), cenotes, ephemeral water bodies, marshes, wet campos, deep-sea hydrothermal vents and on plants as well. Moreover free-living cyclopoid and harpacticoid copepods may form an important component of cryptozoic fauna in moist forest litter (Reid, 1986; Fiers & Ghenne, 2000). Coastal, estuarine brackish waters harbor many copepod genera and species which can persist in these unfavourable habitats mainly as resting, diapausing eggs. Copepods can be found also in strange cryptic habitats, such as the small pools between the leaves of some tropical plants, crab burrows, tree holes and even discarded car tyres (!). Copepods, as many other aquatic organisms, can be easily transported, either actively or passively, often as resistant stages (Sars, 1901; Van de Velde 1984; Matsumura-Tundisi et al. 1990; Saunders et al., 1993; Reid & Reed 1994).
Neverthless, the astonishing success of this group of microcrustaceans is due to their symbiotic relationships with other organisms: in fact, they virtually can parasitize or be the intermediate hosts of all the animal groups, from sponges to vertebrates, including mammalians and man, and it is likely that the number of associated or parasitic taxa known today could represent only a small fraction of the living species, especially in marine waters. Copepods that parasitize fish skin and gills are serious pests of commercial importance in both marine and freshwater fish farms.
Stygobitic copepods originated from surface, marine and freshwater ancestors, and they reached different groundwater habitats mostly through interstitial and crevicular or karstic corridors. Typical groundwater harpacticoid and cyclopoid copepods (Graeteriella, Maraenobiotus, Moraria) seem to occupy large distribution areas, as a consequence of their greater dispersion capabilities as compared to those of macrofaunal subterranean elements (Fiers & Ghenne, 2000).
The systematics of copepods has been subjected to numerous revisions during the last decade and before: at present, according to Huys R.& G.A. Boxshall (1991), ten orders are recognized, half of which (Misophrioida, Monstrilloida, Mormonilloida, Siphonostomatoida, Poecilostomatoida) mostly contains exclusive symbiotic or parasitic species, the others (Platycopioida, Calanoida, Harpacticoida, Gelyelloida, Cyclopoida) as a rule include free-living forms. Brachiura are also included with the Copepoda, since many parasitic copepod researchers also study these parasites of fish, commonly referred to as sea lice. The Class Brachiura is comprised of 1 Family and 4 Genera: Argulus, Chonopeltis, Dipteropeltis, Dolops. 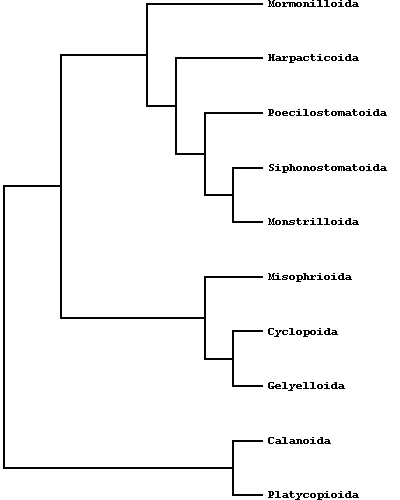 Calanoids, cyclopoids and harpacticoids show a remarkable ecological interest, since most species of these orders generally form the first link of the aquatic food chains, from the microscopic phytoplanktonic algae up to the fishes and mammalians. Recent researches, including those carried out by the Dipartimento di Scienze Ambientali of the University of L'Aquila (Italy), are pointing out an analogous importance for cyclopoid and harpacticoid species inhabiting both surface and underground fresh waters, and particularly the sediments between the superficial hyporheic zone and the rivers bottom, an interesting transitional system or ecotone between epigean and stygal waters. As a matter of fact, the contiguity with surface waters and the regular occurence of epigean elements in the hyporheic habitats let now the hydrobiologists to consider that a good estimation of rivers meiobenthic conditions must pass through a careful knowledge of the relative groundwater communities. In this last regard, an increasing number of harpacticoid and cyclopoid species are actually revealing their noteworthy importance as "pollution markers" in the environmental control of hyporheic systems and other aquatic habitats, such as lakes, springs, rivers and superficial ground waters.

Although the present knowledge of the copepod fauna of Italy, as a whole, is still largely incomplete, subterranean taxa (including anchihaline waters dwellings) are without doubt much better known than surface ones.
Groundwater copepods of Italy are represented by members of the orders Cyclopoida, Harpacticoida, Calanoida and Misophrioida, most of which are stygobitic or stygophilic inhabitants of underground water systems.
They are widely distributed and most successful in nearly all freshwater underground habitats of the country, viz. springs, wells, interstitial media, coastal waters and pools in caves; fresh or brackish coastal waters may often harbor representatives of generally marine taxa, such as Halicyclops and Neocyclops among the cyclopids, and Ectinosoma, Halectinosoma, Pseudectinosoma, Arenopontia and Delamarella among the harpacticoids.
About half of the over 200 known species appear to be endemic to Italy, the remaining ones are for the most part european and palaearctic or they show a wider or cosmopolitan distribution.
A large amount of specialized taxa is found amongst the cyclopids and harpacticoids; on the contrary, the calanoid order, which is very limited in the subterranean realm, is poorly represented also in the ground waters of Italy where a single species, Troglodiaptomus sketi, recently discovered in karstic substrates of Venetia (North Italy), is known. The order Misophrioida, as well, include a single subterranean species, Expansophria sarda, known for anchialine habitats of Sardinia.
Among the cyclopids, the genus Diacyclops is the most diversified and widespread both in continental and insular part of the country, with more than 30 subterranean species/subspecies which concentrate in the Apennine and Sardinian provinces.
T
A remarkable endemism occurs in this genus, which includes a conspicuous number of taxa, such as D. bicuspidatus lucanus, D. crassicaudis var. cosana, D. crassicaudis lagrecai, D. crassicaudis trinacriae, D. cristinae, D. ichnusae, D. languidoides aprutinus, D. lindae, D. maggii, D. nuragicus, D. paolae, D. paralanguidoides, D. ruffoi and D. sardous, with a distribution limited to Italy.
The remarkable stygobitic genera Speocyclops and Graeteriella include less species, as well as they show a distribution limited to the Alpine or central Apennine provinces and Sardinia.
The genus Metacyclops contains, as well, noteworthy stygobitic species, such as M. stammeri and M. subdolus, from South Italy, M. gasparoi and M. trisetosus from Northeastern Italy.
The genera Eucyclops and Acanthocyclops, which are considered primarily epigean genera, harbor also some stygobitic or stygophilic species, endemic to Italy. Other genera (Halicyclops, Neocyclops, Macrocyclops, Paracyclops, Tropocyclops, Cyclops, Thermocyclops, Mesocyclops) regularly live in surface waters and only occasionally they can be found in groundwater networks of Italy, as well as they occur throughout most of the country.
The harpacticoid order is represented in Italy by more stygobitic species, for the most part endemic to the country.
The family Parastenocarididae, which at present contains five recognized genera, viz. Parastenocaris, Forficatocaris, Paraforficatocaris, Potamocaris and the recently described Murunducaris, is represented in Italy only by the most speciose and widespread genus Parastenocaris.
Formerly, this genus was supposed to be represented in Italy only by seven species, sampled in caves (Campania), rivers and lakes (North Italy); later on, due to the considerable attention focused on this genus in recent years, the number of species is remarkably increased, and to date 27 taxa, most endemic, are known from Italy.
Interestingly enough, since the interstitial habitat, although spatially continuum, is rather heterogeneous, both in biotic and chemiophysical factors, some italian species of the nominate genus seem to be particularly affected by changes in environmental characteristics, and to be patchy distributed along the groundwater systems.The distribution of four species of Parastenocaris in the lake sandbanks of central Italy (Lazio) is in agreement with the above hypothesis: three species are more or less homogeneously distributed along the shore interstitial, while P. veneris can be found only in deep ground waters.The endemic P. ima (La Maddalena island, Sardinia) lives only in deep phreatic waters; in fact, this very demanding and specialist taxon was never sampled, although repeated investigations, in superficial ground waters.
Other interesting italian stygobitic or stygophilic harpacticoid species belong to the following families: Ameiridae, with the genera Nitocrella, Parapseudoleptomesochra and Psyllocamptus; Canthocamptidae, with the genera Ceuthonectes, Elaphoidella, Neoelaphoidella and Elaphoidellopsis; Laophontidae with the genus Esola; Ectinosomatidae, with the genera Pseudectinosoma and Ectinosoma; Leptopontidae with the genera Ichnusella and Arenopontia.
Of the 18 and 10 italian species referred to Elaphoidella and Nitocrella respectively, more than 50% are endemic to Italy.
Schizopera, a genus belonging to the family Diosaccidae, which includes species generally living in marine or brackish waters, is well represented in Italy also in continental or costal ground waters.
Different colonization pathways could be pointed out for the italian groundwater copepods, and they are mostly related to different geological and palaeogeographical events pending the late Miocene and Quaternary.
Particularly, some genera, such as Halicyclops, Neocyclops, Nitocrella and Parapseudoleptomesochra could be directly sea-originated, through coastal habitats, according to the "Two-step Model Evolution", and owing to the freshening of coastal interstitial waters following sea regressions or tectonic uplift ; other genera probably reached the continental ground waters from the sea, but through karstic environments or via anchialine caves. Other ones, viz. Speocyclops, Graeteriella, Bryocamptus, Elaphoidella, Moraria and Diacyclops, successfully colonized the continental ground waters from surface fresh water bodies, during different geologic ages, as a result of drastic climatic changes.
The Parastenocarididae, including only true interstitial freshwater species, probably evolved from a marine psammic ancestor, phylogeneticalhy related to Cylindropsyllidae.
As regard the thalassoid forms, we hereby mention only the genera Arenopontia and Delamarella, which were supposed to inhabit exclusively marine and brackish interstitial waters.
The recent discovery of Arenopontia species in continental fresh waters can be explained by the active migration model (Coineau & Boutin, 1993), which can also be in agreement with the occurence, in marine genera as Ectinosoma, Halectinosoma, Arenosetella and Delamarella, of continental groundwater species.
Among the sea-originated taxa, some species of the genera Nitocrella, Parapseudoleptomesochra, and Schizopera could have dispersed actively to previously uncolonized areas due to their high degree of salinity tolerance (Active Migration Model).
The origin of the few italian species of the genera Thermocyclops and Ceuthonectes is still doubtful, since they show discontinuous or isolated distribution.
Genus Acanthocyclops
Acanthocyclops agamus Kiefer 1938 (3) *
Genus Diacyclops
Diacyclops antrincola Kiefer 1967 (1,3)
Genus Eucyclops
Eucyclops graeteri (Chappuis 1927) (2,3)
Genus Graeteriella
Graeteriella unisetigera (Graeter 1908) (1,3)
Genus Halicyclops
Halicyclops dalmatinus Petkovski 1955 (3,5)
Genus Neocyclops
Neocyclops (Protoneocyclops) mediterraneus (Kiefer, 1960) [south Italy; caves,]
Genus Megacyclops
Megacyclops brachypus Kiefer 1954 (2)
Genus Metacyclops
Metacyclops gasparoi Stoch 1987 (1) *
Genus Speocyclops
Speocylops demetiensis (Scourfield 1932) (1)
Family Cyclopinidae
Genus Muceddina
Muceddina multispinosa Jaume & Boxshall 1996 (2)
Family Misophriidae
Genus Expansophria
Expansophria sarda Jaume & Boxshall 1996 (2) *
Family Diaptomidae
Genus Troglodiaptomus
Troglodiaptomus sketi Petkovski 1978 (1)
Family Diosaccidae
Genus Schizopera
Schizopera cicolanii Galassi & Pesce 1988 (4) *
Family Ameiridae
Genus Psyllocamptus
Psyllocamptus monachus (Chappuis 1938) (3) *
Genus Nitocrella
Nitocrella achaiae Pesce 1980 (3,4)
Genus Parapseudoleptomesochra
Parapseudoleptomesochra italica Pesce & Petkovski 1980 (3) *
Family Canthocamptidae
Genus Ceuthonectes
Ceuthonectes serbicus Chappuis 1924 (1)
Genus Elaphoidella
Elaphoidella aprutina Pesce, Galassi & Apostolov 1987 (3) *
Genus Elaphoidellopsis
Elaphoidellopsis dubia (Kiefer 1931) (1) *
Genus Neoelaphoidella
Neoelaphoidella apostolovi Pesce & De Laurentiis 1994 (3) *
Genus Moraria
Moraria denticulata Chappuis 1938 (3) *
Genus Attheyella
Attheyella (Mrazekiella) paranaphtalica Pesce & Galassi 1988 (3,5) *
Genus Bryocamptus
Bryocamptus (Rheocamptus) dentatus Chappuis 1937 (3)
Subgen. Limocamptus
Bryocamptus (Limocamptus) cfr. dacicus (Chappuis 1923) (1)
Genus Echinocamptus
Echinocamptus georgevitchi (Chappuis 1927) (1)
Genus Lessinocamptus
Lessinocamptus caoduroi Stoch 1997 (1) *
Family Leptopontidae
Genus Arenopontia
Arenopontia (Neoleptastacus) phreatica Cottarelli, Bruno & Venanzetti 1994 (2) *
Genus Ichnusella
Ichnusella eione Cottarelli 1971 (2) *
Family Parastenocarididae
Genus Parastenocaris
Parastenocaris acherusia Noodt 1955 (1) *
Family Laophontidae
Genus Esola
Esola spelaea (Chappuis 1938) (3) *
Family Latiremidae
Genus Delamarella
Delamarella galateae Cottarelli 1971 (2,3) *
Family Ectinosomatidae
Genus Ectinosoma
Ectinosoma melaniceps Boeck 1865 (1)
Genus Pseudectinosoma
Pseudectinosoma kunzi Galassi 1997 (3) *
Family Tetragonicipitidae
Genus Phyllopodopsyllus
Phyllopodopsyllus sp. Cottarelli et al., 1996 (1)
[Numbers between brackets according to the stygofaunal provinces of Italy; Checklist of World Groundwater Harpacticoid Copepods
Threatened species in Italy according to IUCN Red List Categories
CHECKLIST OF THE ITALIAN COPEPODS
A selected World bibliography on subterranean copepods can be found in: Lescher-Moutoué F. (1984). Copepoda Cyclopoida Cyclopidae des eaux douces souterraines continentales. Stygofauna Mundi: 299-312; Herbst H.V. (1984). Copepoda:Cyclopoida aus dem Meeres- und Brackwasser-interstitial. Stygofauna Mundi: 313-320; Rouch R. (1984)Copepoda: les harpacticoides souterraines des eaux douces continentales. Stygofauna Mundi: 321-355; Rouch R. (1994). Copepoda. Encyclopaedia Biospeologica. Societé de Biospéologie, Moulis, Bucarest: 105-111.
Other fundamental papers on copepods are those of Dussart B. (H.). (1967). Les Copépodes des eaux continentales d'Europe occidentale. Tome 1: Calanoïdes et Harpacticoïdes. Paris: 1-500; Dussart B. (H.). (1969). Les Copépodes des eaux continentales d'Europe occidentale. Tome 2:Cyclopoïdes et Biologie. Paris:1-292; Kiefer F. (1978). Das Zooplankton der Binnengewässer. Freilebende Copepoda. Die Binnengewässer, Stuttg., 26: 1-343; Dussart B. (H.) & D. Defaye (1990). Répertoire mondial des crustacés copépodes des eaux intérieures. III. Harpacticoïdes. Crustaceana, suppl. 16:1-364; Dussart B. (H.) & D. Defaye (1983). Répertoire mondial des Crustacés Copépodes des eaux intérieures. I. Calanoides. ed. C.N.R.S., Paris, pp.1-224; Dussart B. (H.) & D. Defaye (1985). Répertoire mondial des Copépodes Cyclopoides. ed. C.N.R.S., Paris, pp.1-236; Einsle U. (1993). Crustacea Copepoda Calanoida und Cyclopoida.Subwasserfauna von Mitteleuropa, 8/4-1: 208; Vervoort W. (1986). Bibliography of copepoda, up to and including 1980., 1-3. Crustaceana: 1-1316. Dussart B. & D. Defaye (1995). Copepoda. Introduction to the Copepoda. SPB Academic Publishing bv 1995, pp.1-277
A complete checklist of all the fresh and brackish water copepods of Italy, updated to 1995, is presented on vol. 28 of the "Checklist delle specie della fauna italiana" (1995), by A. Minelli, S. Ruffo & S. La Posta.
The book is available by "Edizioni Calderini", Via Emilia Levante, 31 - Bologna (Italy) (£. 15.000)
VII International Conference on Copepoda
|