The copepods are the largest and most diversified group of crustaceans. At present they include 14.485 species, 2.400 genera and 210 families (more than 2800 are reported from freshwaters) a surely underestimated number, inhabiting sea and continental waters, semiterrestrial habitats, or living in symbiotic relationships with other organisms; about 2.800 species live in fresh waters
(Boxshall and Defaye 2008). They are considered the most plentful multicellular group on the earth, outnumbering even the insects, which include more species, but fewer individuals! Particularly, the copepods are the dominant forms of the marine plankton and constitute the secondary producers in the marine environments and a fundamental step in the trophodinamics of the oceans.
During their long evolutionary history, starting in the Lower Cretaceous, copepods spread over all the continents, as well as they successfully colonized about all the available water habitats of the Planet, becoming well adapted or specialized to very different salinity regimes, from marine and hypersaline waters to continental freshwater bodies, and to a wide range of temperature from the polar to the hot springs waters. Copepods regularly live also in marine and freshwater sediments, in different groundwater substrates, in continental and anchialine caves (limestone caves), cenotes, ephemeral water bodies, bromeliads, marshes, wet campos, and on plants as well. Moreover free-living cyclopoid and harpacticoid copepods may form an important component of cryptozoic fauna in moist forest litter (Reid, 1986, 2001; Fiers & Ghenne, 2000). Coastal, estuarine brackish waters harbor many copepod genera and species which can persist in these unfavourable habitats mainly as resting, diapausing eggs. Copepods can be found also in strange cryptic habitats, such as the small pools between the leaves of some tropical plants, crab burrows, tree holes and even discarded car tyres (!). Copepods, as many other aquatic organisms, can be easily transported, either actively or passively, often as resistant stages (Sars, 1901; Van de Velde 1984; Matsumura-Tundisi et al. 1990; Saunders et al., 1993; Reid & Reed 1994).
Moreover, copepods are the most species-rich and abundant
invertebrates recorded from deep-sea hydrothermal
vents and seeps (Desbruyères & Segonzac
1997; Tsurumi & Tunnicliffe 2001; Gebruk
2002). At present, the list of these copepods, all
reported from the Pacific and Atlantic oceans, includes
81 species (37 genera, 20 families, six orders);
but only a few studies of their biology,
functional morphology, and evolution have been
conducted (Humes & Segonzac 1998; Heptner
& Ivanenko 2002; Tsurumi et al. 2003). The
largest and most widespread family of deep-sea
hydrothermal vent copepods (and invertebrates)
is the siphonostomatoid family Dirivultidae
Humes & Dojiri, 1980 (52 species, 13 genera)
known only from hydrothermal systems (Ivanenko
& Ferrari 2003) (Ivanenko & Defaye, 2004)
Neverthless, the astonishing success of this group of microcrustaceans is due to their symbiotic relationships with other organisms: in fact, they virtually can parasitize or be the intermediate hosts of all the animal groups, from sponges to vertebrates, including mammalians and man, and it is likely that the number of associated or parasitic taxa known today could represent only a small fraction of the living species, especially in marine waters. Copepods that parasitize fish skin and gills are serious pests of commercial importance in both marine and freshwater fish farms.
Some species of cyclopoid genera such as Metacyclops mendocinus, Tropocyclops prasinus, Eucyclops serrulatus, Eucyclops solitarius, Eucyclops ensifer, Macrocyclops albidus and Mesocyclops longisetus
are biological control agents for disease-bearing anopheline mosquitoes, specially as regards the larvae of the dengue vectors Aedes aegypti and Aedes albopictus.
Subterrean copepods originated from surface, marine and freshwater ancestors, and they reached different groundwater habitats mostly through interstitial and crevicular or karstic corridors. Typical groundwater harpacticoid and cyclopoid copepods (Graeteriella, Maraenobiotus, Moraria) seem to occupy large distribution areas, as a consequence of their greater dispersion capabilities as compared to those of macrofaunal subterranean elements (Fiers & Ghenne, 2000). The physical structure of copepods varies greatly, however, the free-living forms of copepods have certain physical traits in common. The body is usually short and cylindrical, composed of a head, thorax, and abdomen. The lower part of the copepod's head is generally fused with its thorax; the front of its head often juts forward, like a tiny beak. Its thorax is divided into about six segments; each segment is connected to two appendages. Generally, copepod has two pair of antennae and a single eye. The first pair of antennae is larger and has bristles. The male copepod can be distinguished from female because its antennae are slightly different from those of the female, modified for holding her during copulation. The free-living copepods limbs are used for movement, sometimes with the help of the antennae. The thin abdomen lacks limbs, except for the caudal furca. There are two basic plans of body organization or tagmosis in copepods, gymnoplean and podoplean, differentiated by the position of the major body articulation. In the gymnoplean plan, this is behind the fifth pedigerous somite whereas in the podoplean plan (Harpacticoida) it is between the fourth and fifth pedigerous somites. The major articulation divides the body into an anterior prosome and a posterior urosome. The prosome is further into two sub-regions. Anteriorly is the cephalosome bearing the five head appendages and the maxillipeds and posterior to this are three free prosomites bearing the second to fourth swimming legs. The urosome comprises an anterior somite bearing the fifth pair of legs and five other somites (referred to as abdomen). 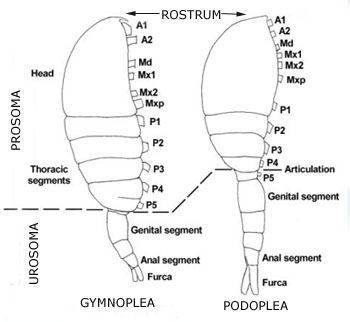 From: Copepods from the Bay of Vilelfranche (www.obs-vlfr.fr/~gaspari/copepods_guide) (modif.)
In the males, all the usoromites are separated but, in the females, the second and third urosomites are usually fused to form the genital double-somite. The last urosomite or anal somite, in which the median anus opens either terminally or dorsally, bears two posterior caudal rami or furcal rami.
The systematics of copepods has been subjected to numerous revisions during the last decade and before. Two infraclasses and nine orders are recognized by Boxshall & Halsey, 2004 and Dussart & Defaye, 2006). The old order Poecilostomatoida has been recently moved to Cyclopoida (Boxshall & Halsey, 2004; Dussart & Defaye, 2006), but Suarez-Morals & Fuentes-Reina (2015) The infraclass Progymnoplea includes a single order, the Platycopioida, the infraclass Neocopepoda contains two superorders: Gymnoplea and Podoplea. The former includes the Calanoida, the latter the remaining seven orders (Misophrioida, Monstrilloida, Mormonilloida, Siphonostomatoida, Harpacticoida, Gelyelloida, Cyclopoida). The Monstrilloida and Siphonostomatoida mostly contains exclusive symbiotic or parasitic species, the others as a rule include free-living forms (Huys & Boxshall,1991).
The Poecilostomatoida is no longer recognised as a valid order; instead its component families lie on a well defined "poecilostome" lineage wholly contained within the Cyclopoida. (from: Khodami et al., 2017). The following orders are represented in groundwater habitats : Platycopioida, Calanoida, Misophrioida, Gelyelloida, Harpacticoida and Cyclopoida.
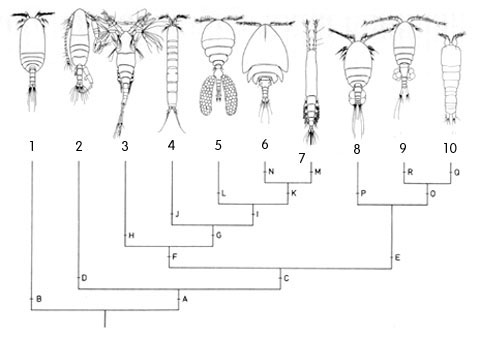
The phylogenetic relationships of copepods have been debated in the last decades. Some authors proposed evolutionary schemes on account of the ecological radiation of copepods (Boxshall, 1986; Ho, 1994) , other authors pointed out cladistic approaches. Particularly Huys & Boxshall (1991) reconstructed the character states of the old ten copepods orders:
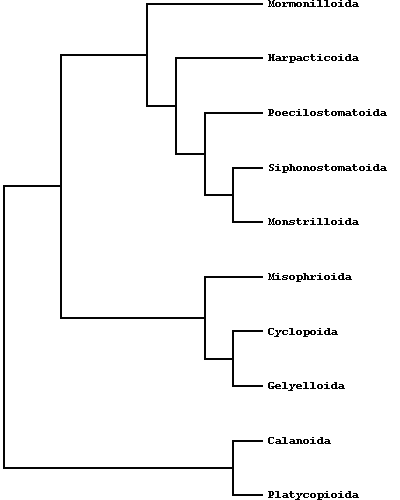
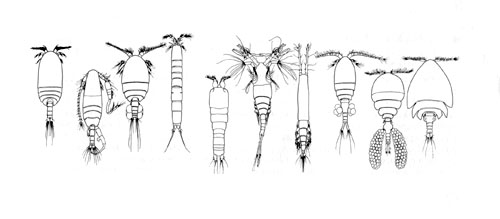
Calanoids, cyclopoids and harpacticoids show a remarkable ecological interest, since most species of these orders generally form the first link of the aquatic food chains, from the microscopic phytoplanktonic algae up to the fishes and mammalians. Recent researches, including those carried out by the Dipartimento di Scienze Ambientali of the University of L'Aquila (Italy), are pointing out an analogous importance for cyclopoid and harpacticoid species inhabiting both surface and underground fresh waters, and particularly the sediments between the superficial hyporheic zone and the rivers bottom, an interesting transitional system or ecotone between epigean and stygal waters. As a matter of fact, the contiguity with surface waters and the regular occurence of epigean elements in the hyporheic habitats let now the hydrobiologists to consider that a good estimation of rivers meiobenthic conditions must pass through a careful knowledge of the relative groundwater communities. In this last regard, an increasing number of harpacticoid and cyclopoid species are actually revealing their noteworthy importance as "pollution markers" in the environmental control of hyporheic systems and other aquatic habitats, such as lakes, springs, rivers and superficial ground (phreatic) waters. Ho et al. (2003) showed that "analysis of the phylogenetic relationships of Thaumatopsyllidae shows that it is not a member of any of the other previously established orders of Copepoda. Accordingly, a new order, Thaumatopsylloida, is proposed to accommodate the five species of thaumatopsyllids thus far reported and is shown to be a member of a Thaumatopsylloida-Monstrilloida-Siphonostomatoida clade", but this opinion is not shared from the most copepodologists. Recently Khodami et al., (2017) investigated the phylogenetic relationships between representatives of all copepod orders using 28S and 18S rRNA, Histone H3 protein and COI mtDNA. The order Canuelloida is proposed to include the families Canuellidae and Longipediidae. 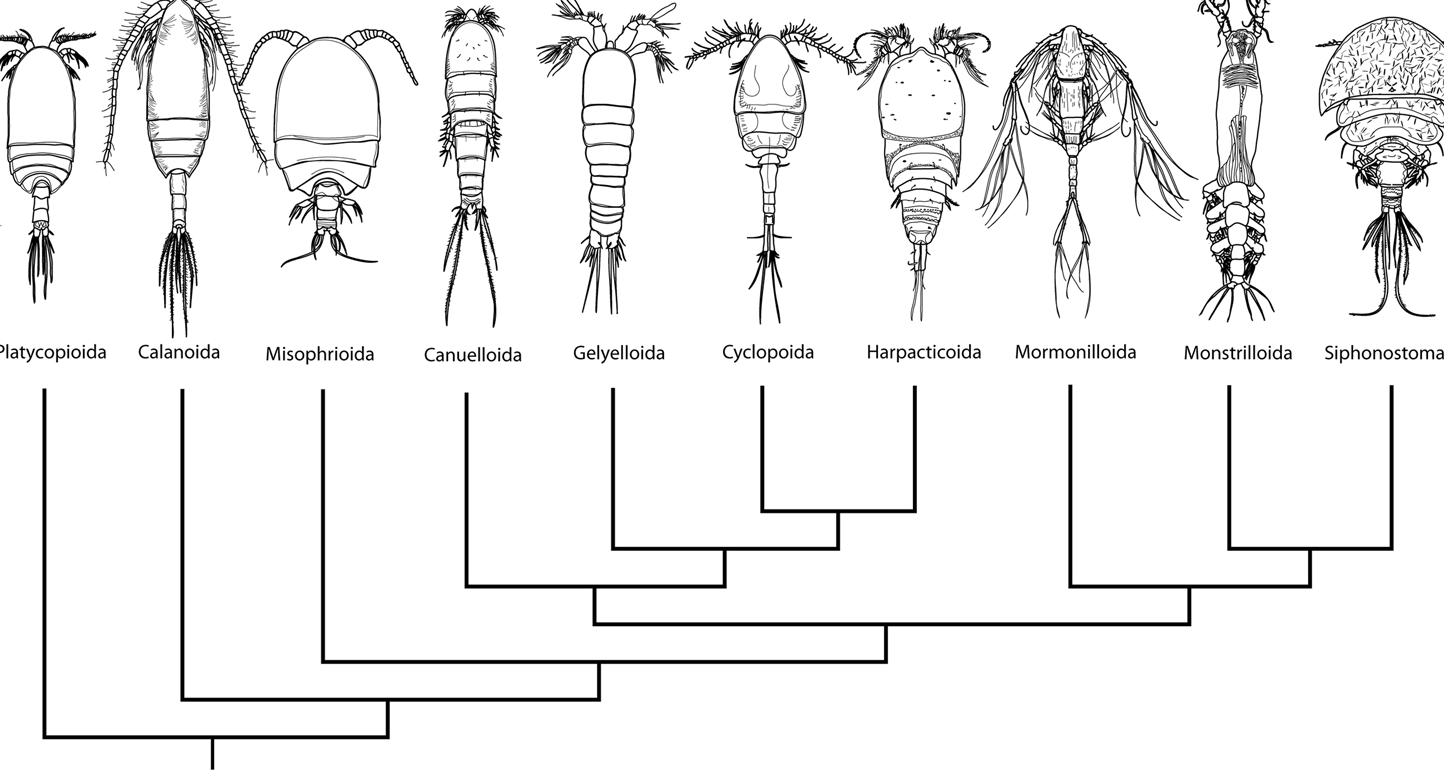 New phylogram of copepod Orders from: Khodami et al., (2017)
According to He et al., 2023 Unresolved cases of type fixation, synonymy and homonymy in
harpacticoid copepod nomenclature (Crustacea: Copepoda) 
The Catalogue of Cyclopoid copepods (Crustacea:
Copepoda: Cyclopidae) from Andriana Damian-
Georgescu Collection (“Grigore Antipa” National
Museum of Natural History, Bucharest, Romania) AN UPDATED CLASSIFICATION OF THE RECENT CRUSTACEA
Conservation of continental copepod crustaceans (Modern approaches to the study of Crustaceans in: Escobar-Briones & Alavarez, 2023)
Conservation of
continental copepod crustaceans |